Arek Socha, Pixabay
Do you have a chronic health problem such as asthma, diabetes or arthritis? In the US, 125 million people (around 38% of the population) suffer from these types of diseases, and treating them takes up 78% of the healthcare budget. The figures are probably similar for other developed countries.
At the Faraday Institute summer course last month, the Oxford-based biologist Paul Fairchild explained that a significant proportion of chronic diseases could be treated by replacing just one of the patient’s cell types or tissues. The use of ‘stem cells’ is a rapidly growing area of research and medicine, but it also throws up some very significant ethical issues. I’ll summarise the main points from his lecture here, in the hope of equipping others to start exploring this area and have a voice in the public arena about the development and use of these new treatments.
Most of the cells in our bodies are specialised: blood, bone marrow, liver, and so on. Stem cells are generalists which can turn into a number of different cell types. In medicine, the more types of cell a stem cell can become, the more useful it is.
One particular type of flatworm has become famous among biologists for being able to regenerate all its tissues at any point in its lifecycle. This feat is possible because the worm has ‘pluripotent’ stem cells that are capable of turning into any type of cell in its body.
By the time a human embryo has implanted in its mother’s womb it has lost the flatworm’s amazing regenerative abilities. But if cells from a pre-implantation embryo – perhaps one that is ‘left over’ after IVF treatment – are kept under the right laboratory conditions, they will multiply almost indefinitely. These Embryonic Stem (ES) cells can be treated to become different tissue types, and have been used to help patients with diabetes, cardiovascular disease, macular degeneration and a number of different neurological conditions.
The potential to heal people using ES cells is enormous, but it throws up a very serious ethical question. What is the status of a preimplantation embryo? Does it deserve the same rights and protection as a newborn baby, or does it deserve respect but not the same moral status as an adult? Some think that embryos develop their unique personhood over time, as they get past the stage when an embryo can divide into identical twins, start to develop a nervous system, or become aware of their surroundings. Others, including Paul, think a person comes into existence at conception, but this view needn’t necessarily by a show-stopper for ES cell research.
If an embryo conceived in an IVF clinic is not used within 5 years, it must be destroyed. Paul and many other scientists would prefer to use its ES cells, and some even see it as morally wrong not to use these unwanted embryos to develop life-saving treatments. Others would prefer not to benefit from a process they believe shouldn’t be happening (i.e. discarding human embryos).
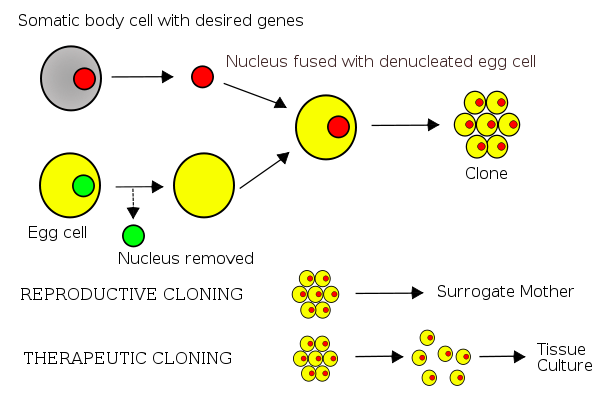
To get round the problem of tissue matching (which is as much an issue with an ES cell transplants as with other types of tissue transplants), scientists have also developed ‘therapeutic cloning’. For example, if I needed some new heart cells doctors could take an egg cell from my own body (if I were a man they would need to source a egg cell from a donor) and take out the DNA. This first step is important because eggs and sperm only contain half the amount of DNA needed to make full-functioning cells. The next step would be to add the full set of DNA from another cell in my body, and then treat that new hybrid cell in the lab to make it multiply and produce ES cells. Those cells could then be turned into heart cells, which would be transplanted back into my body.
Dr. Jürgen Groth/ Belkorin/Wikibob, Creative Commons Attribution-Share Alike 3.0 Unported license.
One ethical issue with therapeutic cloning, apart from the expense, is hinted at in the name. Once my DNA was inserted into the egg it could, if used as it would be in an IVF clinic, become a person. I could give birth to my own clone, which is illegal worldwide. This alternative scenario also shows that a potentially viable embryo is destroyed as part of therapeutic cloning, and many people object to that.
Another cell-based treatment involves making ‘induced pluripotent cells’ (iPSCs). To treat my fictitious heart problem, cells could be taken from my body and treated to make them pluripotent again. This is the procedure that Paul investigates in his own lab. It’s not straightforward, but once the treatment has been figured out it is very powerful. iPSCs can be used not just to treat disease, but to test new drugs or study the disease process.
iPSCs still don’t solve all the ethical dilemmas because the same process can be used to make eggs or sperm – or even both, raising the spectre of human cloning again. iPSC technology can also be used to make whole new organs, but these need to be made in a host animal. If someone grew a new heart for me in a pig, my cells might not only be found in the organ we wanted but in other parts of its body – including the gametes and brain, raising questions about what it means to be human.
Every new technology can promise both great good and potential harm. The use of stem cells may offer powerful solutions to chronic disease, but the ethical questions they raise are as complex as the procedures themselves. It could be tempting to jettison this whole area of medicine just to be on the safe side, but I suspect that would not be a wise move. I would rather stay informed, have my say in the public discussion, and support scientists such as Paul who are attempting to navigate the field as wisely as he can.
For further exploration
You can listen to the full recording of Paul Fairchild’s lecture here.
Prof Paul Fairchild, Thinking about stem cells (pdf)
Dr Peter Moore, Thinking about bioethics (pdf)
A variety of views on stem cells from previous talks at the Faraday Institute.




