If you ask a 14 year old, an 18 year old and an undergraduate to describe an atom you will get different answers. Ask them to draw an atom, and the discrepancies become even more noticeable. A 14 year old will have no issues producing an image like the one below. The undergraduate is likely to look at you quizzically. “Draw an atom? You must be joking!”
Chemistry, much like the other sciences, relies upon models. As students progress through their chemistry education they are introduced to increasingly complex models of the atom. Each model is used to explain the various chemical observations that students encounter.
From age 11 to 16, students work with an atomic model that can provide an (adequate) explanation of why sodium chloride’s structure is composed of positive sodium ions and negative chlorine ions in a 1:1 ratio. But it fails to provide an explanation for the beautiful colours of transition metal complexes such as haemoglobin. The simplified atomic model cannot account for the different colours of oxygenated and de-oxygenated blood. As the model begins to fail, we are forced to reconsider our model and develop it.
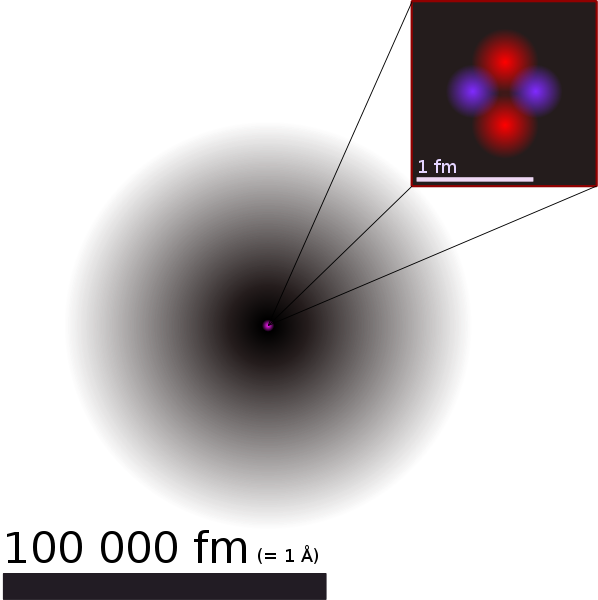
By Helium_atom_QM.svg: User:Yzmo derivative work: Cepheiden (Helium_atom_QM.svg) [GFDL or CC-BY-SA-3.0], via Wikimedia Commons
So what, you might ask, does this have to do with belief in God?
As a Christian and a trained chemist, I am interested in the pursuit of truth and reality. Yet I am often forced to recognize that the real truth and the true reality may in fact be difficult to fully comprehend, and I reach for models to help me explain and grasp these concepts. None of the models are wrong. But neither are they complete. Every model captures an essence of reality.
When it comes to understanding and talking about God, we make comparisons: God is like this or like that. One could go so far as to say we are limited to models of God. The reality of God is surely much more complex than our understanding.

Holy Trinity by Lawrence OP. Flickr. (CC BY-NC-ND 2.0)
As a Christian, I believe in the three persons of the Godhead often called the Trinity. I believe in one God, whilst affirming that the Father, Son and the Holy Spirit are distinct. Believing in an apparent contradiction may seem irrational. But through chemistry I’ve become familiar with the wave-particle duality of an electron. Sometimes an electron seems to act like a particle. Sometimes it seems to act like a wave. There is nothing from my everyday experience that is remotely similar to wave-particle duality. It seems contradictory and yet, scientific experimentation suggests otherwise.
In organic chemistry, I used to write out reaction mechanisms using curly arrows to represent the movement of a pair of electrons. It was problem solving with pictures, and we used a simplified understanding of chemical bonding. However, quite remarkably we could use the method to predict the product that would be formed from a complicated chemical reaction.
In inorganic chemistry, I studied molecular orbital theory that looked at bonding within compounds in a lot more detail. This often seemed incompatible with the simplistic model we used when working out organic reaction mechanisms. Being an inorganic chemist, I naturally also thought it was superior. The models had huge explanatory power but were useful for different problems – I needed to know when to use each model.

(c) FreeFoto.com. (CC BY-NC-ND 3.0)
It might come as a surprise that we do this in theology as well. Christians all agree that something unique happened when Jesus died on the cross. He atoned for our sin: through his death our sins can be forgiven. How exactly he achieved that feat can often be a subject of debate. Throughout history, the church has taught various ‘atonement theories,’ or we could call them atonement models. Unfortunately, our natural tendency is to emphasize one of these models to the exclusion of others, seeing one as the ultimate truth. Each model of the atonement has explanatory power, adds to our understanding and calls us to worship. However, each model is not without its difficulties or limitations. Apply a model too literally and we will likely end up with heresy. Appreciate its limitations, and the model can be helpful.

Holy Cross-Immaculata Church By Nheyob (Own work) [CC BY-SA 4.0], via Wikimedia Commons
The essence of God is difficult to capture in a model, yet Jesus dares us to believe we can speak with confidence about the nature of God. Jesus said to his followers, ‘Anyone who has seen me, has seen the Father.’ As Paul, an author of the New Testament exclaimed, ‘He (Jesus) is the image of the invisible God.’
Since God is inherently complex to understand, Jesus is the person of the Trinity I find easiest to grasp. And if I can speak with a degree of poetic license, he is the simplified model that I can begin with.

Joe Ogborn is a chemistry graduate, musician, teacher, writer, and member of staff at City Church, Cambridge. He is really interested in the interplay between Christianity, productivity and creativity.





